Acetylenic scaffolding with subphthalocyanines – synthetic scope and elucidation of electronic interactions in dimeric structures†
Abstract
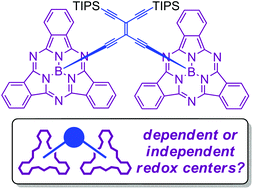
Boron subphthalocyanines (SubPcs) are powerful chromophoric heterocycles that can be synthetically modified at both axial and peripheral positions. Acetylenic scaffolding offers the possibility of building large, unsaturated carbon-rich frameworks that can exhibit excellent electron-accepting properties, and when combined with SubPcs it presents a convenient method for preparing interesting chromophore–acceptor architectures. Here we present synthetic methodologies for the post-functionalization of the relatively sensitive SubPc chromophore via acetylenic coupling reactions. By gentle AlCl3-mediated alkynylation at the axial boron position, we managed to anchor two SubPcs to the geminal positions of a tetraethynylethene (TEE) acceptor. Convenient conditions that allow for stepwise desilylations of trimethylsilyl (TMS) and triisopropylsilyl (TIPS) protected SubPc-decorated acetylenes using silver(I) fluoride were developed. The resulting terminal alkynes were successfully used as coupling partners in metal-catalyzed couplings, providing access to larger acetylenic SubPc scaffolds and multiple chromophore systems. Moreover, conditions allowing for the conversion of a terminal alkyne into an iodoalkyne in the presence of SubPc were developed, and the product was subjected to cross-coupling reactions affording unsymmetrical 1,3-butadiynes. The degree of interactions between two SubPc units as a function of the acetylenic bridge was studied by UV-Vis absorption spectroscopy and cyclic voltammetry. A TEE bridging unit was found to strongly influence the reductions and oxidations of the two SubPc units, while a more flexible bridge had no influence.


 Please wait while we load your content...
Please wait while we load your content...