Why can a gold salt react as a base?†
Abstract
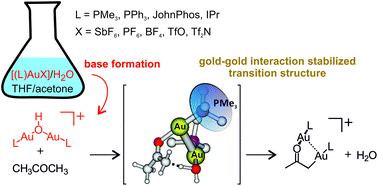
This study shows that gold salts [(L)AuX] (L = PMe3, PPh3, JohnPhos, IPr; X = SbF6, PF6, BF4, TfO, Tf2N) act as bases in aqueous solutions and can transform acetone to digold acetonyl complexes [(L)2Au2(CH2COCH3)]+ without any additional base present in solution. The key step is the formation of digold hydroxide complexes [(L)2Au2(OH)]+. The kinetics of the formation of the digold complexes and their mutual transformation is studied by electrospray ionization mass spectrometry and the delayed reactant labelling method. We show that the formation of digold hydroxide is the essential first step towards the formation of the digold acetonyl complex, the reaction is favoured by more polar solvents, and the effect of counter ions is negligible. DFT calculations suggest that digold hydroxide and digold acetonyl complexes can exist in solution only due to the stabilization by the interaction with two gold atoms. The reaction between the digold hydroxide and acetone proceeds towards the dimer {[(L)Au(OH)]·[(L)Au(CH3COCH3)]+}. The monomeric units interact at the gold atoms in the perpendicular arrangement typical of the gold clusters bound by the aurophilic interaction. The hydrogen is transferred within the dimer and the reaction continues towards the digold acetonyl complex and water.
- This article is part of the themed collection: Mechanistic Aspects of Organic Synthesis


 Please wait while we load your content...
Please wait while we load your content...