Cerium-catalyzed, oxidative synthesis of annulated, tetrasubstituted dihydrofuran-derivatives†
Abstract
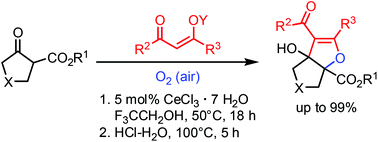
Densely functionalized, annulated dihydrofuran derivatives are prepared by a cerium-catalyzed aerobic oxidation reaction. The operationally simple transformation is environmentally and economically benign, since the precatalyst CeCl3·7H2O is non-toxic and inexpensive and the oxidant is simply dioxygen from air. Starting materials are β-oxoesters and silylenolethers, and the latter are derived from acetoacetate or acetylacetone. The reaction sequence is performed in one flask and consists of α-oxidation and Mukaiyama aldol reaction. Apart from tetrahydrocyclopenta[b]furan derivatives one example of a tetrahydrofuro[3,4-b]furan and one tetrahydro-3aH-furo[2,3-c]pyrrole derivative are prepared.


 Please wait while we load your content...
Please wait while we load your content...