Boronic acid recognition of non-interacting carbohydrates for biomedical applications: increasing fluorescence signals of minimally interacting aldoses and sucralose†
Abstract
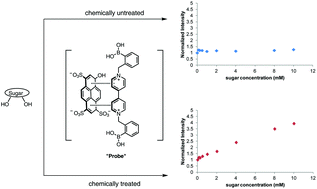
To address carbohydrates that are commonly used in biomedical applications with low binding affinities for boronic acid based detection systems, two chemical modification methods were utilized to increase sensitivity. Modified carbohydrates were analyzed using a two component fluorescent probe based on boronic acid-appended viologen–HPTS (4,4′-o-BBV). Carbohydrates normally giving poor signals (fucose, L-rhamnose, xylose) were subjected to sodium borohydride (NaBH4) reduction in ambient conditions for 1 h yielding the corresponding sugar alcohols from fucose, L-rhamnose and xylose in essentially quantitative yields. Compared to original aldoses, apparent binding affinities were increased 4–25-fold. The chlorinated sweetener and colon permeability marker sucralose (Splenda), otherwise undetectable by boronic acids, was dechlorinated to a detectable derivative by reactive oxygen and hydroxide intermediates by the Fenton reaction or by H2O2 and UV light. This method is specific to sucralose as other common sugars, such as sucrose, do not contain any carbon–chlorine bonds. Significant fluorescence response was obtained for chemically modified sucralose with the 4,4′-o-BBV–HPTS probe system. This proof of principle can be applied to biomedical applications, such as gut permeability, malabsorption, etc.
- This article is part of the themed collection: Chemosensors and Molecular Logic


 Please wait while we load your content...
Please wait while we load your content...