The trimerization of acetylenes involves a cascade of biradical and pericyclic processes†
Abstract
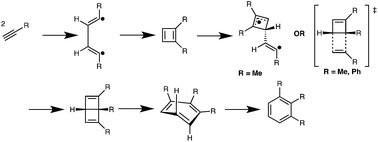
Thorough computational studies were performed on mechanisms and energies for the thermal trimerizations of neutral or electron-rich acetylenes used as cross-linkers in organic hard-masks for lithography applications. These studies indicate that the operative mechanism proceeds through initial cyclobutadiene formation via a biradical mechanism. Cyclobutadienes form Dewar benzenes via Diels–Alder cycloadditions, or biradical processes, or both, before producing benzenes by electrocyclic ring-opening reactions. These pathways are preferred to alternatives involving concerted trimerizations or mechanisms involving carbene intermediates.
- This article is part of the themed collection: Mechanistic Aspects of Organic Synthesis


 Please wait while we load your content...
Please wait while we load your content...