Synthesis of oligonucleotides containing 2-N-heteroarylguanine residues and their effect on duplex/triplex stability†
Abstract
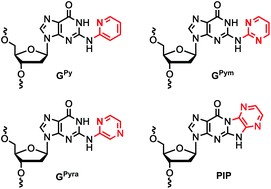
To systematically understand the effect of 2-N-heteroarylguanine (GHA) modification on the stability of higher-order DNA structures, nucleoside derivatives and oligodeoxyribonucleotides containing guanine residues modified with four kinds of hereroaryl groups on the 2-amino group were synthesized. The stabilities of the DNA duplex and the parallel-oriented DNA triplex containing these GHAs were studied by measuring their melting temperatures (Tm). Tm experiments and DFT calculations of the modified guanine nucleobases suggested that the base pair formation energy and stability of the two conformations, i.e., the open- and closed-type conformations, are key to determining the stability of the DNA duplex. Finally, the DNA triplex was destabilized when modified guanine residues were introduced into triplex-forming oligonucleotides.


 Please wait while we load your content...
Please wait while we load your content...