Exploring the origins of selectivity in soluble epoxide hydrolase from Bacillus megaterium†
Abstract
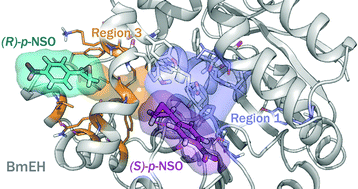
Epoxide hydrolase (EH) enzymes catalyze the hydration of racemic epoxides to yield their corresponding vicinal diols. These enzymes present different enantio- and regioselectivity depending upon either the substrate structure or the substitution pattern of the epoxide ring. In this study, we computationally investigate the Bacillus megaterium epoxide hydrolase (BmEH)-mediated hydrolysis of racemic styrene oxide (rac-SO) and its para-nitro styrene oxide (rac-p-NSO) derivative using density functional theory (DFT) and an active site cluster model consisting of 195 and 197 atoms, respectively. Full reaction mechanisms for epoxide ring opening were evaluated considering the attack at both oxirane carbons and considering two possible orientations of the substrate at the BmEH active site. Our results indicate that for both SO and p-NSO substrates the BmEH enantio- and regioselectivity is opposite to the inherent (R)-BmEH selectivity, the attack at the benzylic position (C1) of the (S)-enantiomer being the most favoured chemical outcome.
- This article is part of the themed collection: Mechanistic Aspects of Organic Synthesis


 Please wait while we load your content...
Please wait while we load your content...