Copper(i)-catalyzed 5-exo-trig radical cyclization/borylation of alkyl halides: access to functionalized pyrrolidine derivatives†
Abstract
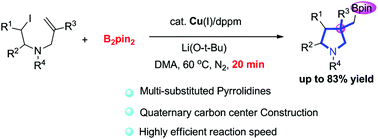
This work reports the copper(I)-catalyzed 5-exo-trig radical cyclization/borylation of alkyl halides bearing an alkene moiety, during which a C–C bond and a C–B bond were formed in one step. Various functionalized pyrrolidine derivatives bearing a quaternary carbon center were obtained, and they showed good functional group tolerance and high chemoselectivity. This transformation was highly efficient and could be finished in 20 minutes. A radical mechanism has been proposed.


 Please wait while we load your content...
Please wait while we load your content...