Computational characterization of the mechanism for the light-driven catalytic trichloromethylation of acylpyridines†
Abstract
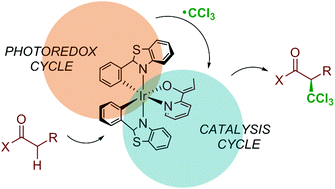
The computational characterization of the mechanism for complex reactions involving the photoactivation of transition metal compounds remains a challenge for theoretical chemistry. In this work we show how the application of DFT and ONIOM(DFT:MM) methods can characterize the photoinduced iridium-catalyzed enantioselective trichloromethylation of 2-acylpyridines that was recently reported by Meggers and co-workers. This is a complex process, as it involves two linked catalytic cycles and yields the product with high enantioselectivity. Calculations succeed in reproducing all available experimental data, including the sign and value of the enantiomeric excess. The detailed mechanistic picture that is obtained leads to the identification of the origin of selectivity as the steric repulsion between an attacking trichloromethyl radical and the ligands at iridium in the path leads to the minor enantiomer.
- This article is part of the themed collection: Mechanistic Aspects of Organic Synthesis


 Please wait while we load your content...
Please wait while we load your content...