Phosphine-catalyzed intramolecular Rauhut–Currier reaction: enantioselective synthesis of hydro-2H-indole derivatives†
Abstract
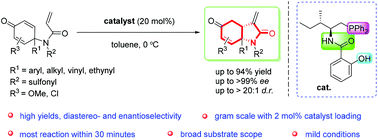
A highly enantioselective intramolecular Rauhut–Currier reaction catalyzed by a multifunctional chiral aminophosphine catalyst was reported. A series of hydro-2H-indole derivatives that bear an all-carbon quaternary center were obtained in high yields (up to 94%), and excellent diastereo- and enantioselectivities (up to >20 : 1 dr and >99% ee). And this reaction could be performed on a gram scale using 2 mol% catalyst loading.


 Please wait while we load your content...
Please wait while we load your content...