18O2 labeling experiments illuminate the oxidation of ent-kaurene in bacterial gibberellin biosynthesis†
Abstract
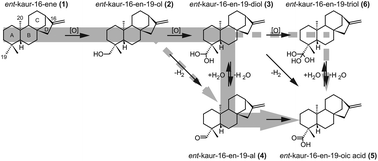
Bacteria can produce gibberellin plant hormones. While the bacterial biosynthetic pathway is similar to that of plants, the individual enzymes are very distantly related and arose via convergent evolution. The cytochromes P450 (CYPs) that catalyze the multi-step oxidation of the alkane precursor ent-kaurene (1) to ent-kauren-19-oic acid (5), are called ent-kaurene oxidases (KOs), and in plants are from the CYP701 family, and share less than 19% amino acid sequence identity with those from bacteria, which are from the phylogenetically distinct CYP117 family. Here the reaction series catalyzed by CYP117 was examined by 18O2 labeling experiments, the results indicate successive hydroxylation of 1 to ent-kauren-19-ol (2) and then ent-kauren-19,19-diol (3) and most likely an intervening dehydration to ent-kauren-19-al (4) prior to the concluding hydroxylation to 5. Accordingly, the bacterial and plant KOs converged on catalysis of the same series of reactions, despite their independent evolutionary origin.


 Please wait while we load your content...
Please wait while we load your content...