Total syntheses of smenothiazoles A and B†
Abstract
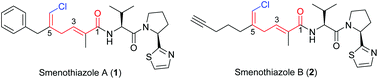
Concise total syntheses of smenothiazoles A (1) and B (2), two distinguished vinyl chloride containing natural products isolated from the marine sponge S. aurea, have been developed. Silastannation, Stille reaction and a carefully controlled desilylchlorination were employed as key steps to construct unique polyketide acid fragments, and the optimized reaction conditions avoided migration of 2,5-diene to a 2,4-conjugated system. This report unambiguously confirmed the structures of both natural products.


 Please wait while we load your content...
Please wait while we load your content...