Novel pyrrolobenzodiazepine and pyrroloquinazoline scaffolds synthesized by a simple and highly selective Ugi/cyclization sequence†
Abstract
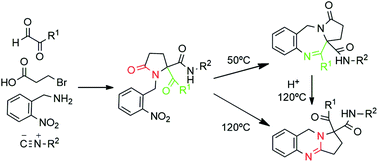
Pyrrolo[2,1-c][1,4]benzodiazepines (PBDs) and other benzo-fused N-heterocycles constitute privileged structures found in numerous bioactive compounds. Thus, developing simple and selective syntheses to furnish these derivatives from easily accessible starting materials is an important and challenging goal. In this work, novel pyrrolobenzodiazepine and pyrroloquinazoline derivatives have been synthesized following a common two step synthetic strategy. This strategy involves a one-pot Ugi/cyclization sequence followed by a reduction with spontaneous thermocontrolled cyclization. The control of the temperature in this second step allows fully selective access to either pyrrolo[2,1-c][1,4]benzodiazepine-3-ones 6 or pyrrolo[2,1-b]quinazolines 7. Density functional theory (DFT) calculations have been carried out to rationalize this reactivity, identifying the kinetic and thermodynamic reaction products and offering insights into the cyclization pathways. These synthetic methodologies show the versatility of the Ugi reaction as a tool in the synthesis of heterocyclic compounds with a pseudopeptidic skeleton.


 Please wait while we load your content...
Please wait while we load your content...