BODIPY dyads from a,c-biladiene salts†
Abstract
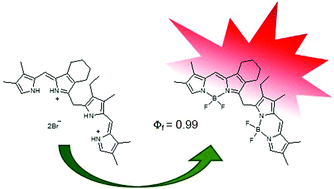
Asymmetric dimers of BODIPY dyes were synthesized from a,c-biladiene salts in good yields; this work constitutes a new versatile approach to the synthesis of BODIPY dyads, which display red-shifted absorptions and emissions in the visible spectral region, higher fluorescence quantum yields and larger Stokes shifts compared with monomeric BODIPYs. The X-ray structure of a 5,5′-dibromo-BODIPY dyad was obtained, and the reactivity of this compound under Suzuki cross-coupling reaction conditions was investigated.


 Please wait while we load your content...
Please wait while we load your content...