Oxidative C–H functionalization of N-carbamoyl 1,2-dihydroquinolines†
Abstract
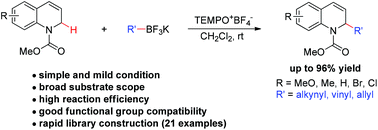
A modular and efficient method for the synthesis of α-substituted 1,2-dihydroquinolines is described. Under mild metal-free conditions, readily available N-carbamoyl 1,2-dihydroquinolines undergo oxidative C–H alkynylation, alkenylation, and allylation with a range of potassium trifluoroborates using TEMPO oxoammonium salt as an oxidant.


 Please wait while we load your content...
Please wait while we load your content...