Synthesis of 2-aminoBODIPYs by palladium catalysed amination†
Abstract
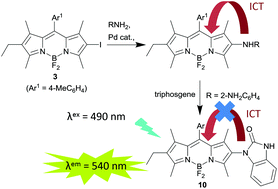
Palladium catalysed coupling of the 2-iodoBODIPY 3 with a range of anilines and a primary alkylamine succeeds in generating the corresponding 2-aminoBODIPYs. These 2-aminoBODIPY derivatives are non-emissive and quantum chemical calculations and electrochemistry are consistent with charge transfer from the amine substituent. Attenuation of this charge transfer pathway by conversion of the 1,2-phenylenediamine derivative 9 into the corresponding benzimidazolone 10 restores the fluorescence and has been used as the basis for a fluorescence sensor for phosgene.


 Please wait while we load your content...
Please wait while we load your content...