One-flask synthesis of pyrazolone thioethers involving catalyzed and uncatalyzed thioetherification pathways of pyrazolones†
Abstract
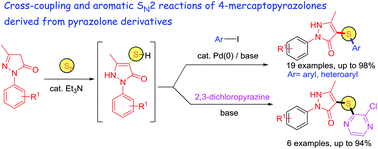
A one-flask thioetherification of pyrazolones has been demonstrated by transforming several pyrazolones to their corresponding 4-mercaptopyrazolone derivatives and employing them towards cross-coupling with various aromatic and heteroaromatic iodides by applying Pd(OAc)2/xantphos as the catalytic system. The coupling ability of these thiol intermediates with 2,3-dichloropyrazine through an aromatic SN2 pathway has also been established. This methodology provides the use of inexpensive starting materials along with a short reaction time.


 Please wait while we load your content...
Please wait while we load your content...