Dinuclear zinc complex catalyzed asymmetric methylation and alkynylation of aromatic aldehydes†
Abstract
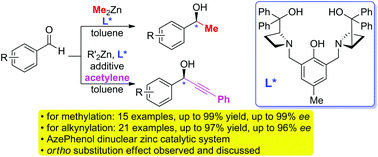
A general AzePhenol dinuclear zinc catalytic system has been successfully developed and introduced into the asymmetric addition of dimethylzinc and alkynylzinc to aromatic aldehydes. In this system, an azetidine derived chiral ligand has proven to be an effective enantioselective promoter. Under the optimal reaction conditions, a series of chiral 1-hydroxyethyl (up to 99% ee) and secondary propargylic alcohols (up to 96% ee) were generated with good yields and enantioselectivities. Additionally, this novel catalytic system showed good functional group compatibility. Remarkably, the substituent's electronic nature alone is not sufficient to allow for exclusive enantioselectivity, an additional substituent's location also had an effect. We proposed that the formation of a stable and structural rigid transition state by the chelation of ortho substituted benzaldehydes to the zinc atom was responsible for the observed higher enantioselectivity. The possible catalytic cycles of both transformations accounting for the stereoselectivity were described accordingly.


 Please wait while we load your content...
Please wait while we load your content...