Formation of hydrazones and stabilized boron–nitrogen heterocycles in aqueous solution from carbohydrazides and ortho-formylphenylboronic acids†
Abstract
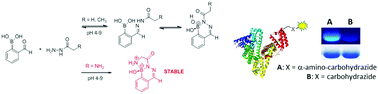
A recent addition to the suite of fast bioorthogonal reactions combines hydrazines and hydroxylamines with ortho-carbonyl substituted phenylboronic acids. Carbohydrazides are easily incorporated into biomolecules, making them appealing substrates in these reactions. Here we show that simple alkyl carbohydrazides form a single product with ortho-formylphenylboronic acid in an organic solvent and in the solid state. The solution structures of the products formed from the carbohydrazides in buffered aqueous solution, however, are markedly different from those identified in the organic solvent and solid state. The reactants form a mixture of hydrazone and heterocyclic products, the relative composition of which varies with pH. The observed reversibility of bioconjugates using carbohydrazide can thus be explained by the reversibility of the boron–nitrogen bond in the heterocycle. In contrast, the inclusion of an α-amine into the carbohydrazide substrate yields a single product in which both nitrogens are bonded to boron. These tricyclic structures are the same in organic solvent, solid state and aqueous solution from pH 4 to pH 9. Bioconjugates formed with α-amino carbohydrazides are stable to SDS-PAGE, while those formed with simple alkyl carbohydrazides are not. We propose that the inclusion of an intramolecular stabilizing ligand into a carbohydrazide substrate is a generally applicable principle that may be exploited to form boronic acid-based bioconjugates with a defined structure and resistance to hydrolysis.


 Please wait while we load your content...
Please wait while we load your content...