A one-pot synthesis of 2,2′-disubstituted diindolylmethanes (DIMs) via a sequential Sonogashira coupling and cycloisomerization/C3-functionalization of 2-iodoanilines†
Abstract
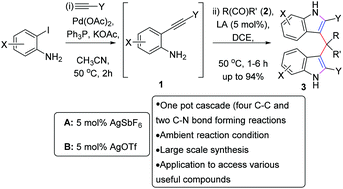
A Pd(II)–Ag(I) catalyzed highly efficient synthesis of diindolylmethane has been developed. This transformation consists of a one-pot sequential Sonogashira coupling (and desilylation) followed by cycloisomerization/C3-functionalization of 2-iodoanilines. Six new bonds (four C–C and two C–N) are formed in a one-pot fashion. A variety of diindolylmethanes were obtained in excellent yields (up to 94%) under mild reaction conditions and this strategy is amenable to gram scale synthesis also. The products were transformed into various synthetically useful compounds.


 Please wait while we load your content...
Please wait while we load your content...