Photochemical generation and trapping of 3-oxacyclohexyne†
Abstract
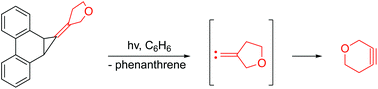
The strained heterocyclic alkyne, 3-oxacyclohexyne, was generated photochemically for the first time using a cyclopropanated phenanthrene precursor, and trapped by cyclopentadienones as Diels–Alder adducts. The precursor initially produced the putative 3-oxacyclopentylidenecarbene that subsequently rearranged to the cycloalkyne. Computational studies indicate that the carbene favors a singlet state, and the barrier for its ring expansion by a 1,2-shift of the carbon proximal to oxygen is lower in energy than the corresponding shift of the distal carbon.
- This article is part of the themed collection: Mechanistic Aspects of Organic Synthesis


 Please wait while we load your content...
Please wait while we load your content...