Advances in chemoselective intermolecular cross-benzoin-type condensation reactions
Abstract
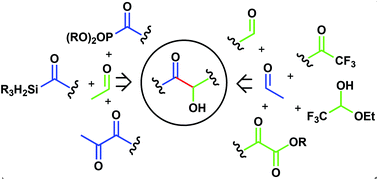
The intermolecular cross-benzoin and acyloin condensation reactions are powerful approaches to α-hydroxy carbonyls in a single step. However, their potentiality suffers from the occurrence of side reactions including self-condensation and the formation of the undesired cross-acyloin. The broad range of azolium salt precatalysts available confers high tunability to NHC mediated benzoin condensation, assuring a good level of selectivity to the direct coupling between two non-equivalent aldehydes. Many efforts have also been devoted to the design of strategies that expand the range of suitable reaction partners beyond the traditional aldehydes and to the discovery of novel umpolung catalytic systems. The synthesis of both racemic and enantiomerically enriched acyloins is reviewed.
- This article is part of the themed collection: Celebrating excellence in research: women of organic chemistry


 Please wait while we load your content...
Please wait while we load your content...