A reevaluation of the origin of the rate acceleration for enzyme-catalyzed hydride transfer
Abstract
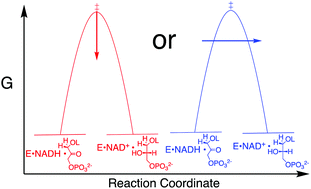
There is no consensus of opinion on the origin of the large rate accelerations observed for enzyme-catalyzed hydride transfer. The interpretation of recent results from studies on hydride transfer reactions catalyzed by alcohol dehydrogenase (ADH) focus on the proposal that the effective barrier height is reduced by quantum-mechanical tunneling through the energy barrier. This interpretation contrasts sharply with the notion that enzymatic rate accelerations are obtained through direct stabilization of the transition state for the nonenzymatic reaction in water. The binding energy of the dianion of substrate DHAP provides 11 kcal mol−1 stabilization of the transition state for the hydride transfer reaction catalyzed by glycerol-3-phosphate dehydrogenase (GPDH). We summarize evidence that the binding interactions between (GPDH) and dianion activators are utilized directly for stabilization of the transition state for enzyme-catalyzed hydride transfer. The possibility is considered, and then discounted, that these dianion binding interactions are utilized for the stabilization of a tunnel ready state (TRS) that enables efficient tunneling of the transferred hydride through the energy barrier, and underneath the energy maximum for the transition state. It is noted that the evidence to support the existence of a tunnel-ready state for the hydride transfer reactions catalyzed by ADH is ambiguous. We propose that the rate acceleration for ADH is due to the utilization of the binding energy of the cofactor NAD+/NADH in the stabilization of the transition state for enzyme-catalyzed hydride transfer.
- This article is part of the themed collection: Mechanistic Aspects of Organic Synthesis


 Please wait while we load your content...
Please wait while we load your content...