Synthesis of γ-keto sulfones by copper-catalyzed oxidative sulfonylation of tertiary cyclopropanols†
Abstract
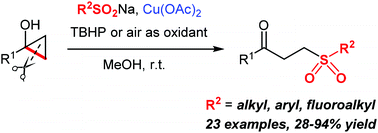
Tertiary cyclopropanols undergo ring-opening oxidative sulfonylation to afford γ-keto sulfones when reacting with sulfinate salts in the presence of a copper(II) acetate catalyst and an oxidant (tert-butyl hydroperoxide or atmospheric oxygen). Various fluoroalkyl, aryl and alkyl sulfinate salts are successfully employed as sulfonylation reagents, affording the corresponding sulfones in up to 94% yields. The experimental protocol is mild and tolerates a number of functionalities in the cyclopropanol substrate. The reaction proceeds via a one-pot oxidation–Michael addition mechanism and can serve as a useful addition to the existing methods for the preparation of γ-keto sulfones based on the sulfa-Michael reaction.


 Please wait while we load your content...
Please wait while we load your content...