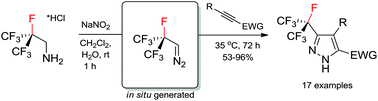
Heptafluoroisopropyl diazomethane (i-C3F7CHN2): in situ generation and synthesis of pyrazoles†
Abstract
A novel chemical reagent – heptafluoroisopropyl diazomethane (i-C3F7CHN2) – has been generated in situ in aqueous media. It readily reacts with electron-deficient alkynes towards polyfluorinated pyrazoles that are important in agrochemistry.


 Please wait while we load your content...
Please wait while we load your content...