Metal-free radical aromatic carbonylations mediated by weak bases†
Abstract
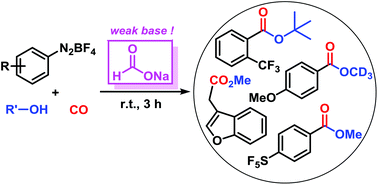
We report a new method of metal-free alkoxycarbonylation. This reaction involves the generation of aryl radicals from arenediazonium salts by a very weak base (HCO2Na) under mild conditions. Subsequent radical trapping with carbon monoxide and alcohols gives alkyl benzoates. The conditions (metal-free, 1 equiv. base, MeCN, r.t., 3 h) tolerate various functional groups (I, Br, Cl, CF3, SF5, NO2, ester). Mechanistic studies indicate the operation of a radical aromatic substitution mechanism.


 Please wait while we load your content...
Please wait while we load your content...