Synthesis of 3-acylindoles by oxidative rearrangement of 2-aminochalcones using a hypervalent iodine reagent and cyclization sequence†
Abstract
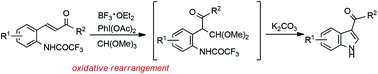
An efficient one-pot method was developed for the construction of 3-acylindoles via oxidative rearrangement of 2-aminochalcones followed by intramolecular cyclization. The reaction was used to convert a variety of 2-aminochalcones into 3-acylindoles in moderate to high yields.


 Please wait while we load your content...
Please wait while we load your content...