Ru-Catalysed synthesis of fused heterocycle-pyridinones and -pyrones†
Abstract
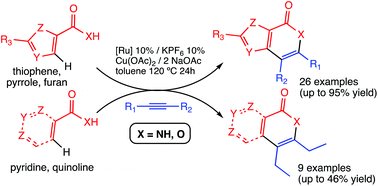
The synthesis of fused heterocycle-pyridinones has been achieved by oxidative coupling of N-unprotected primary heterocycle-amides with internal alkynes. The reaction, which is catalysed by Ru(II) and assisted by Cu(II), takes place through C–H and N–H bond activation of the heterocyclic unit. The scope of the reaction includes a variety of alkynes, electron-rich thiophenes, furans and pyrroles, and even electron-poor pyridines. The reaction is fully regioselective with respect to the position of the C–H bond activation due to the directing effect of the amide group. In the same way, the synthesis of fused heterocycle-pyrones (isocoumarins) has been developed by Ru-catalysed oxidative coupling of heterocyclic carboxylic acids and internal alkynes. The reaction involves C–H and O–H bond activation. This reaction also has a broad scope, from electron-rich thiophenes, furans and pyrroles to electron-deficient pyridines and quinolines.


 Please wait while we load your content...
Please wait while we load your content...