Synthesis of pyrrolidine-3-carboxylic acid derivatives via asymmetric Michael addition reactions of carboxylate-substituted enones†
Abstract
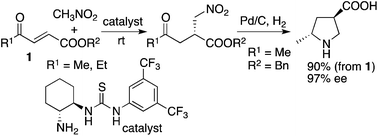
To concisely synthesize highly enantiomerically enriched 5-alkyl-substituted pyrrolidine-3-carboxylic acids, organocatalytic enantioselective Michael addition reactions of 4-alkyl-substituted 4-oxo-2-enoates with nitroalkanes have been developed. Using the developed reaction method, 5-methylpyrrolidine-3-carboxylic acid with 97% ee was obtained in two steps.


 Please wait while we load your content...
Please wait while we load your content...