Carbazole-based BODIPYs with ethynyl substituents at the boron center: solid-state excimer fluorescence in the VIS/NIR region†
Abstract
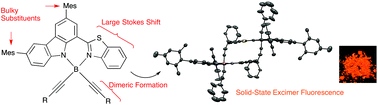
Carbazole-based BODIPYs 1–6 with several different substituents at the boron atom site were synthesized. These dyes fluoresced in the solid state, and 3a with phenylethynyl groups exhibited a red-shifted and broad fluorescence spectrum, which suggested an excimer emission. Its derivatives 3b–n were synthesized, and the relationship between the solid-state emission and crystal packing was investigated. The X-ray crystal structures revealed cofacial dimers that might form excimers. From the structural optimization results, we found that the introduction of mesityl groups hindered intermolecular access and led to reduced interactions between the dimers. In addition, the red-shifted excimer fluorescence suppressed self-absorption, and dyes with ethynyl groups showed solid-state fluorescence in the vis/NIR region.


 Please wait while we load your content...
Please wait while we load your content...