Total syntheses of gerberinol I and the pterophyllins 2 and 4 using the Casnati–Skattebøl reaction under different conditions†
Abstract
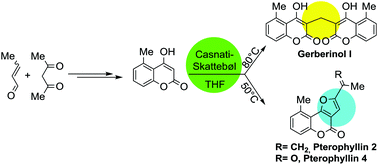
The concise and efficient total syntheses of the naturally-occurring coumarin derivatives gerberinol I, and the pterophyllins 2 and 4, from 5-methyl-4-hydroxycoumarin as a common precursor employing different Casnati–Skattebøl reaction conditions, are reported. The synthesis of the key intermediate coumarin was achieved by the organocatalytic condensation of acetylacetone and crotonaldehyde followed by a LiCl-assisted cyclization, CuCl2-promoted aromatization and a final Et2CO3-mediated cyclization. A Casnati–Skattebøl formylation under high-temperature conditions afforded gerberinol I, whereas milder conditions resulted in an unstable 3-formyl-4-hydroxycoumarin derivative, which was subjected to a basic alumina-mediated one pot O-alkylation with chloroacetone and intramolecular aldolization to furnish pterophyllin 4. Wittig methylenation of the latter conveniently afforded pterophyllin 2.


 Please wait while we load your content...
Please wait while we load your content...