Nucleophile dependent formation of 6- and 7-membered N-heterocycles by platinum-catalysed cyclisation of 1,5-bisallenes†
Abstract
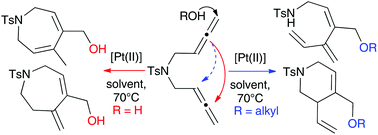
An unprecedented Pt-catalysed cyclisation of N-tethered 1,5-bisallenes in the presence of oxygen nucleophiles is reported, where formation of 6- or 7-membered rings is driven by the choice of nucleophile and the mechanism dictated by the nucleophile and the electronic properties of the bisallene. The reaction in the presence of alcohols gives preferentially vinyltetrahydropyridines with an extra alkoxy group and Pt-H as the active species in the catalytic cycle, while formation of di- and tetrahydroazepines with an extra hydroxyl group is favoured when water is used as nucleophile, via nucleophilic attack/carbocyclization as the favoured pathway. The products obtained are frequently found in the core of natural products with important biological activities, so understanding this complex mechanistic behaviour and exploiting this new methodology will have a big impact in organic synthesis and organometallic chemistry.
- This article is part of the themed collection: Mechanistic Aspects of Organic Synthesis


 Please wait while we load your content...
Please wait while we load your content...