Tetrasubstituted cyclopentadienones as suitable enantiopure ligands with axial chirality†
Abstract
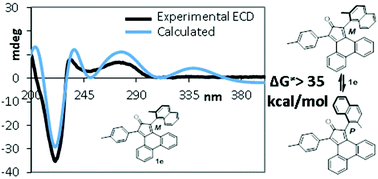
A series of thermally stable atropisomeric phencyclone ligands (ΔG‡rac > 35 kcal mol−1), bearing two chiral axes, has been successfully synthesized, taking into account the results of DFT calculations on model systems. The absolute configurations of the novel atropisomers have been assigned using TD-DFT simulation of ECD spectra. Atropisomeric phencyclones herein presented pave the way towards new ruthenium-based enantioselective hydrogenation catalysts.


 Please wait while we load your content...
Please wait while we load your content...