Phenothiazine-linked nucleosides and nucleotides for redox labelling of DNA†
Abstract
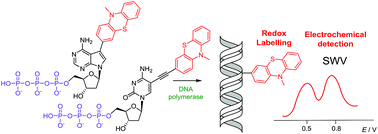
Nucleosides and 2′-deoxyribonucleoside triphosphates (dNTPs) bearing phenothiazine (PT) attached to a nucleobase (cytosine or 7-deazaadenine) either directly or through an acetylene linker were prepared through Suzuki or Sonogashira cross-coupling and triphosphorylation, and were studied as building blocks for polymerase construction of modified DNA. The directly PT-substituted dNTPs were better substrates for polymerases than the alkyne-linked dNTPs but all of them were used in enzymatic synthesis of DNA using primer extension, nicking enzyme amplification, PCR or 3′-tail labelling by terminal deoxynucleotidyl transferase. The phenothiazine served as an oxidizable redox label (giving two analytically useful signals of oxidation on electrode) for nucleosides and DNA and was also used in orthogonal combination with previously developed benzofurazane or nitrophenyl labels for redox coding of DNA bases. Therefore, the title PT-linked dNTPs are useful additions to the portfolio of nucleotides for enzymatic synthesis of redox-labelled DNA for electrochemical analysis.
- This article is part of the themed collection: Nucleic Acid Modifications


 Please wait while we load your content...
Please wait while we load your content...