Palladium-catalyzed oxidative coupling of arylboronic acid with isocyanide to form aromatic carboxylic acids†
Abstract
A valuable palladium-catalyzed oxidative coupling of aryl- and alkenyl borides with isocyanide for the synthesis of corresponding carboxylic acids has been developed. With wide substrate scopes and good functional group tolerance, this reaction offers corresponding carboxylic acids in moderate to excellent yields.
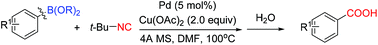


 Please wait while we load your content...
Please wait while we load your content...