Total synthesis of (−)-kainic acid and (+)-allo-kainic acid through SmI2-mediated intramolecular coupling between allyl chloride and an α,β-unsaturated ester†
Abstract
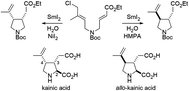
A 3,4-disubstituted pyrrolidine ring was effectively cyclized through SmI2-mediated reductive coupling between allyl chloride and an α,β-unsaturated ester, although little has been reported about SmI2-promoted C–C bond formation of an allyl chloride with an α,β-unsaturated ester. Selection of either the 3,4-cis- or 3,4-trans-selective cyclization can be accomplished simply by changing the additives from NiI2 to HMPA during reductive cyclization conducted in H2O–THF. Total synthesis of (−)-kainic acid and (+)-allo-kainic acid, which are pyrrolidine alkaloids used in neuroscience and neuropharmacology as useful molecular probes, was successfully achieved by using the stereo-complementary ring closure reactions promoted by SmI2 for the construction of the 2,3,4-trisubsituted pyrrolidine scaffold of kainoids.


 Please wait while we load your content...
Please wait while we load your content...