Abstract
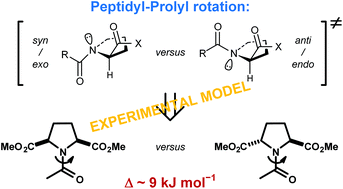
Amide rotation of peptidyl–prolyl fragments is an important factor in backbone structure organization of proteins. Computational studies have indicated that this rotation preferentially proceeds through a defined transition-state structure (syn/exo). Here, we complement the computational findings by determining the amide bond rotation barriers for derivatives of the two symmetric proline analogues, meso and racemic pyrrolidine-2,5-dicarboxylic acids. The rotations around these residues represent syn/exo–syn/exo and anti/endo–syn/exo hybrid transition states for the meso and racemic diastereomer, respectively. The rotation barriers are lower for the former rotation by about 9 kJ mol−1 (aqueous medium), suggesting a strong preference for the syn/exo (clockwise) rotation over the anti/endo (anticlockwise) rotation. The results show that both hybrid rotation processes are enthalpically driven but respond differently to solvent polarity changes due to the different transition state dipole–dipole interactions.


 Please wait while we load your content...
Please wait while we load your content...