Incorporation of ‘click’ chemistry glycomimetics dramatically alters triple-helix stability in an adiponectin model peptide†
Abstract
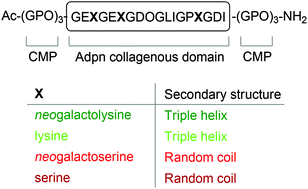
Adiponectin (Adpn) has been shown to be a possible therapeutic for Type II diabetes, however the production of a therapeutic version of Adpn has proved to be challenging. Biological studies have highlighted the importance of the glycosylated lysine residues for the formation of bioactive high molecular weight oligomers of Adpn. Through the use of ‘click’ glycopeptide mimetics, we investigated the role of glycosylated lysine and serine residues for the formation of triple helical structures of the collagenous domain of Adpn, in the context of a collagen model peptide scaffold. The physical properties of the unglycosylated lysine and serine peptides are compared with their glycosylated analogues. Our results highlight the crucial role of lysine residues for formation of the triple helical structure of Adpn, possibly due to the extension of both intra- and interstrand hydrogen bonding networks. Strikingly, we observed a significant decrease in thermal stability upon incorporation of triazole-linked analogues of glycosylated lysine residues into the adiponectin collageneous domain, indicating possible uses of ‘click’ glycomimetics for bioengineering applications.


 Please wait while we load your content...
Please wait while we load your content...