Inhibitors of nicotinamide N-methyltransferase designed to mimic the methylation reaction transition state†
Abstract
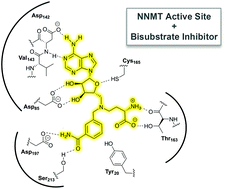
Nicotinamide N-methyltransferase (NNMT) is an enzyme that catalyses the methylation of nicotinamide to form N′-methylnicotinamide. Both NNMT and its methylated product have recently been linked to a variety of diseases, suggesting a role for the enzyme as a therapeutic target beyond its previously ascribed metabolic function in detoxification. We here describe the systematic development of NNMT inhibitors derived from the structures of the substrates involved in the methylation reaction. By covalently linking fragments of the NNMT substrates a diverse library of bisubstrate-like compounds was prepared. The ability of these compounds to inhibit NNMT was evaluated providing valuable insights into the structural tolerances of the enzyme active site. These studies led to the identification of new NNMT inhibitors that mimic the transition state of the methylation reaction and inhibit the enzyme with activity on par with established methyltransferase inhibitors.


 Please wait while we load your content...
Please wait while we load your content...