An expeditious method to synthesize difluoroboron complexes of β-keto amides from β-keto nitriles and BF3·OEt2†
Abstract
A convenient and expeditious strategy to synthesize difluoroboron complexes of β-keto amides has been developed from β-keto nitriles and BF3·OEt2. BF3·OEt2 serves as both a BF2 source and a Lewis acid catalyst in the synthetic strategy. The formation mechanism of the difluoroboron complexes from β-keto nitriles and BF3·OEt2 was proposed. The difluoroboron complexes can be further converted into β-keto amides by treatment with sodium acetate. The strategy features advantages such as a wide substrate scope, non-metal catalysis, and easy operation. Some of the difluoroboron complexes display good fluorescence properties in the solid state and potential application in solid-state luminescent materials.
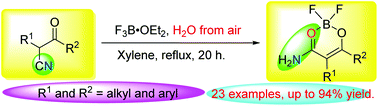


 Please wait while we load your content...
Please wait while we load your content...