Regioselective Diels–Alder reaction to open-cage ketolactam derivatives of C60†
Abstract
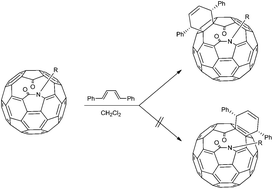
Open-cage ketolactam fullerenes reacted with dienes on the rim of the orifice both regio- and stereoselectively. Unequivocal evidence for the structure of the Diels–Alder adduct was provided by 2D INADEQUATE 13C NMR studies on 13C enriched material, as well as via DFT-GIAO calculations. The theoretical calculations successfully model the regioselective and the endo stereoselective reaction, predicting molecular orbital control along with a repulsive steric interaction between the substituents on the nitrogen atom and those on the diene.


 Please wait while we load your content...
Please wait while we load your content...