Transfer hydrogenation of ortho-hydroxybenzophenone ketimines catalysed by BINOL-derived phosphoric acid occurs by a 14-membered bifunctional transition structure†
Abstract
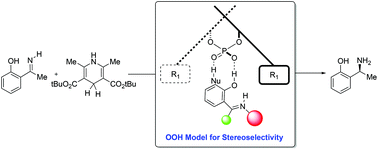
Chiral BINOL-derived phosphoric acids catalyse the transfer hydrogenation of ketimines using Hantszch esters. In many cases the nitrogen on the imine binds to the catalyst through the catalyst hydroxyl group and the nucleophile forms a second hydrogen bond to the phosphoryl oxygen. DFT and ONIOM calculations show that the introduction of an ortho-hydroxyaryl group on the carbon atom of the ketimine leads the reaction to proceed through a 14-membered bifunctional mechanism. The transition states of these reactions involve both hydrogen bonding from the hydroxyl group on the imine and the nucleophile's proton to the phosphate catalyst. This mechanistic pathway is lower in energy than the conventional route, consistent with the experimentally observed increased rates of reaction relative to imines that are not derived from ortho-hydroxybenzophenone. To complement the high-level calculations, an accessible qualitative model has been developed that predicts the correct sense of stereoinduction for all examples.


 Please wait while we load your content...
Please wait while we load your content...