Synthesis of selectively 4-substituted 9,9′-spirobifluorenes and modulation of their photophysical properties†
Abstract
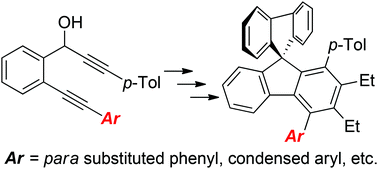
Synthesis of selectively 4-substituted 9,9′-spirobifluorenes was accomplished by using catalytic [2 + 2 + 2]-cyclotrimerization of specifically substituted diynols with alkynes to the corresponding fluorenols. Further synthetic transformations provided the target molecules. The measurement of the photophysical properties of the prepared 4-substituted 9,9′-spirobifluorenes revealed that their emission maxima depended on the electronic properties of the substituents present in the para position: the presence of an electron accepting group strongly favored the maxima red shift toward the blue VIS region (CF3λmax = 361 nm vs. MeO λmax = 330 nm). Adding further substituents (aryl or arylethynyl moieties) on the phenyl ring in position 4 did not lead to a dramatic improvement in the emission maxima (CF3C6H4, λmax = 369 nm, CF3C6H4C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C, λmax = 370 nm), but increased their quantum yields considerably. In addition, a series of 9,9′-spirobifluorenes possessing various extended π-systems (pyrene, anthracene, etc.) were synthesized. In general, the emission maxima pattern reflected that of the parent π-systems, but they were red shifted by 10–30 nm. Finally, also a 1-[4-(9,9′-spirobifluorene-4-yl)phenyl]-2-aryl-ortho-carborane was prepared. These data thus may provide guidelines for further design of 9,9′-spirobifluorenes with tailored properties.
C, λmax = 370 nm), but increased their quantum yields considerably. In addition, a series of 9,9′-spirobifluorenes possessing various extended π-systems (pyrene, anthracene, etc.) were synthesized. In general, the emission maxima pattern reflected that of the parent π-systems, but they were red shifted by 10–30 nm. Finally, also a 1-[4-(9,9′-spirobifluorene-4-yl)phenyl]-2-aryl-ortho-carborane was prepared. These data thus may provide guidelines for further design of 9,9′-spirobifluorenes with tailored properties.


 Please wait while we load your content...
Please wait while we load your content...