C–ON bond homolysis of alkoxyamines: when too high polarity is detrimental†
Abstract
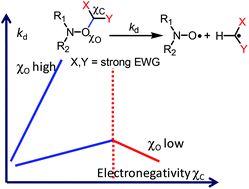
Throughout the last decade, the effect of electron withdrawing groups (EWGs) has been known to play a role – minor or moderate depending on the nitroxyl fragment R1R2NO – in the change in the homolysis rate constant (kd) for C–ON bond homolysis in alkoxyamines (R1R2NOR). It has been shown that the effect of EWGs on kd is described by a linear relationship with the electrical Hammett constant σI. Since then, linear multi-parameter relationships f(σRS,ν,σI) have been developed to account for the effects involved in the changes in kd, which are the stabilization of the released radical (σRS) and the bulkiness (ν) and polarity (σI) of the alkyl fragment. Since a decade ago, new results have been published highlighting the limits of such correlations. In this article, previous multi-parameter linear relationships are amended using a parabolic model, i.e. (σI,nitroxide − σI,alkyl)2, to describe the effect of EWGs in the alkyl fragment on kd. In contrast to previous studies, these improved linear multi-parameter relationships f(σRS,ν,ΔσI2) are able to account for the presence of several EWGs on the alkyl fragment, R. An unexpectedly strong solvent effect – a ca. 1500-fold increase in kd – from tert-butylbenzene to the water/methanol mixture is also observed for 3-((2,2,6,6-tetramethylpiperidin-1-yl)oxyl)pentane-2,4-dione 1b in comparison to a ca. 5-fold increase in kd that is generally observed.


 Please wait while we load your content...
Please wait while we load your content...