Selective construction of polycyclic spirooxindoles via a Cu(OTf)2/HOTf-catalyzed domino reaction of o-arylalkynylacetophenones and 3-phenacylideneoxindoles†
Abstract
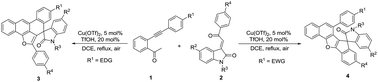
Under the combined catalysis of Cu(OTf)2/HOTf, the domino annulation reaction of o-arylalkynyl acetophenones with 3-phenacylideneoxindoles in refluxing acetonitrile selectively afforded functionalized spiro[indoline-3,7′-tetrapheno[7,6-bc]furans] and spiro[indeno[1,2-b]naphtho[2,1-d]furan-7,3′-indolines] depending on the electronic effect of the substituents on both substrates.


 Please wait while we load your content...
Please wait while we load your content...