Enzymatic competition and cooperation branch the caerulomycin biosynthetic pathway toward different 2,2′-bipyridine members†
Abstract
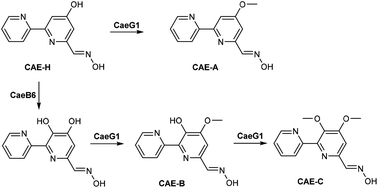
In this study, we characterized CaeB6 as a selective hydroxylase and CaeG1 as an O-methyltransferase in the biosynthesis of the 2,2′-bipyridine natural products caerulomycins (CAEs). The C3-hydroxylation activity of CaeB6 competes with the C4-O-methylation activity of CaeG1 and thereby branches the CAE pathway from a common C4-O-demethylated 2,2′-bipyridine intermediate. CaeG1-catalyzed C4-O-methylation leads to a main route that produces the major product CAE-A in Actinoalloteichus cyanogriseus NRRL B-2194. In contrast, CaeB6-catalyzed C3-hydroxylation results in a shunt route in which CaeG1 causes C4-O-methylation and subsequent C3-O-methylation to produce a series of minor CAE products. These findings provide new insights into the biosynthetic pathway of CAEs and a synthetic biology strategy for the selective functionalization of the 2,2′-bipyridine core.


 Please wait while we load your content...
Please wait while we load your content...