Stereodivergent synthesis of all the four stereoisomers of antidepressant reboxetine†
Abstract
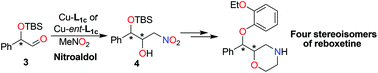
Chiral amino alcohol–copper(II) catalysts Cu-L1c and Cu-ent-L1c were utilized to promote the diastereoselective nitroaldol reactions of chiral aldehydes (S)-3 or (R)-3 with nitromethane, which respectively led to the preferential formation of certain stereoisomer for nitro diol derivatives 4. Using this catalytic protocol, all the four stereoisomers of the antidepressant reboxetine were divergently prepared. The highest overall yield of this synthetic route reached up to 30.5% from aldehyde (S)-3.


 Please wait while we load your content...
Please wait while we load your content...