Governing effects in the mechanism of the gold-catalyzed cycloisomerization of allenic hydroxylamine derivatives†‡
Abstract
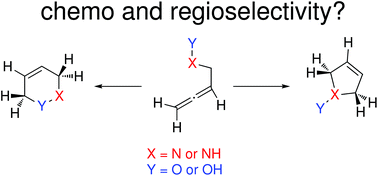
The formation of chiral heterocycles via cycloisomerization reactions of allene derivatives has gained relevance due to their associated efficiency and atom-economy. The only drawback that keeps these reactions away from being routine synthetic strategies is the control in the regioselectivity (most often 5-endo vs. 6-endo). In this work, we computationally explore the experimental chemistry reported by Krause using N-hydroxy-α-aminoallenes and hydroxylamine ethers as substrates and provide a rationale for the different reactivity observed. The drastic effects observed experimentally when changing the nature of the gold catalyst have also been studied mechanistically. These results are expected to help in the design of improved regioselective protocols for the formation of medium sized chiral heterocycles from allene substrates.


 Please wait while we load your content...
Please wait while we load your content...