Synthesis and antiviral evaluation of base-modified deoxythreosyl nucleoside phosphonates†
Abstract
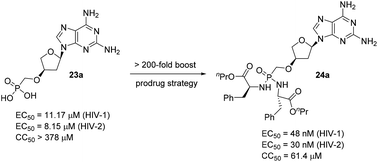
L-α-2′-Deoxythreosyl nucleoside phosphonates and their phosphonodiamidate prodrugs with a hypoxanthine, 2,6-diaminopurine, 2-amino-6-cyclopropylaminopurine, 7-deazaadenine, 5-fluorouracil and 5-methylcytosine heterocycle as a nucleobase were synthesized and evaluated for their inhibitory activity against HIV and HBV. The 2,6-diaminopurine modified analogue 23a displayed the most potent activity against HIV, with an EC50 value of 11.17 μM against HIV-1 (IIIB) and an EC50 value of 8.15 μM against HIV-2 (ROD). The application of the prodrug strategy on nucleoside phosphonate 23a led to a 200-fold boost in anti-HIV potency. None of the compounds showed any activity against HBV at the highest concentration tested.


 Please wait while we load your content...
Please wait while we load your content...