Copper-catalyzed synthesis of indolyl diketones via C–H oxidation/diacylation of indoles with arylglyoxal hydrates†
Abstract
An expedient protocol for Cu-catalyzed C–H oxidation/diacylation of indoles with arylglyoxal hydrates to construct indolyl diketones is developed. This methodology exhibits the synthetic utility of the synthesis of an indole-alkaloid 1,2-di(1H-indol-3-yl)ethane-1,2-dione and offers a straightforward means to produce different indolyl nitrogen-containing heterocycles such as indolyl quinoxaline, indolyl hydantoin and indolyl imidazole in high yields. Preliminary mechanistic studies indicate that two proposed pathways are involved in this process.
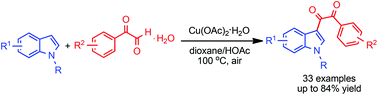


 Please wait while we load your content...
Please wait while we load your content...